Bases:
Bases are metal oxides and metal Hydroxides.
A base is a a substance, that can accept H+ ions and therefore are proton acceptor.
- Copper(II) Oxide (CuO)
- Iron(III) Oxide (Fe2O3)
- Copper (II) Hydroxide (Cu(OH)2)
- Iron (III) Hydroxide (Fe2O3)
It reacts with an acid to give salt and water only.
Neutralization Reaction:
Neutralization reaction occurs, when Acid and Base react to form Metal Salt and Water.
Alkali:
An Alkali is a base, that is soluble in water.
Lets see what happens when an alkali (Sodium Oxide) is added to water
Examples of Alkalis:
- Sodium Hydroxide (NaOH)
- Potassium Hydroxide (KOH)
- Calcium Hydroxide (Ca(OH)2
- Barium Hydroxide (Ba(OH)2
- Aqueous Ammonia (NH3)
Properties:
- Alkalis have bitter taste and soapy feeling.
- Turn Red litmus papers Blue.
- Produce Hydroxide ions when dissolved in H2
e.g.
- All alkalis can react with acids to form a salt and water. This reaction is called neutralization. In this reaction the hydrogen ions from the acid and the hydroxide ions from the alkali react to form water.
Ionic Equation:
- Alkalis when heated with ammonium salts give off ammonia gas.
- Alkalis can react with a solution of one metal salt to give metal hydroxide and another metal salt. The general equation for this reaction is:
- The Metal OH appears as a precipitate if it is insoluble in water.
Strong Alkalis:
Strong Alkali is that, which completely ionizes in solution.
Weak Alkalis:
Weak Alkali is that, which partially ionizes in solution.
Example:
Ammonium Hydroxide (Ammonia gas dissolved in water).–
Distinguishing between weak and strong Alkali:
Stronger Alkali at the same concentration has higher pH.
At the same concentration, stronger alkali would be the best conductor of electricity.
Uses of Basis And Alkalis:
- Ammonium Solution:
- In window cleaning solution
- In fertilizers
Calcium Oxide:
- In neutralizing acidic soil.
- To make Iron, Concrete and Cement.
Magnesium Hydroxide:
- In toothpaste to neutralize acid on teeth.
- In antacids, to relieve indigestion.
Sodium Hydroxide:
- In making soaps and detergents.
- Industrial – Cleaning detergents.
p H scale:
The p H scale is a set of numbers used to indicate whether a solution is acidic, neutral or alkaline.
p H Calculation:
Based on number of H ions and OH ions:
Strong Acids – Higher concentration of H ions.
Strong Alkalis – Higher concentration of OH ions.
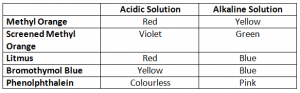
Indicators:
Why is Soil pH important?
- It affects the growth and development of plants.
- Plants best grow when the soil is neutral or slightly acidic.
- Plants will not grow in soil that is too acidic.
- This can happen when too much fertilizer is added to the soil and due to acid rain.
Hence the pH value of soil should be between 5 and 9.
Controlling Acidity of Soil:
- Chemicals are often added when soil becomes too acidic.
- The soil is treated with bases such as:
Quick Lime (Calcium Oxide) CaO
Slaked Lime (Calcium Hydroxide) CaOH
Lime Stone (Calcium Carbonate) CaCO3
- The basis react with the acids in the soil and raise the p H so that plants can grow healthily.
However adding too much base will make soil too much alkaline – thus making soil unsuitable for crop growth.
Oxides:
Oxides are compounds containing Oxygen.
Acidic Oxides:
- Non Metals may form acidic oxides.
- Most acidic oxides dissolve in water to form acids.
- They do not react with acids but they react with alkalis to form a salt and water.
Examples:
Neutralization Reaction:
Basic Oxides:
- The oxides of metal are basic oxides.
- Most Basic Oxides are insoluble in water.
- Those that are soluble are called alkalis ( Na2O)
- Solid at room temperature.
- React with acids to form a salt and water.
- No reaction with bases or alkalis.
- In soluble in bases.
Examples:
Amphoteric Oxides:
- Metal Oxides that react with both acids and basis to form salt and water.
- Oxides that contain both the abilities of acids and alkalis.
- If atmospheric oxide is reacted with an acid it will show the properties of an alkali and a neutralization reaction occurs.
- If it is reacted with an alkali – will shows acidic properties – a neutralization reaction occurs.
- Showing reaction with both acids and base.
- Oxides of Aluminum (Al), Zinc(Zn) and Lead(Pb).
- Produce Salt and H2O when reacting with acid or alkali.
Example:
Neutral Oxides:
- Some Non – Metals form oxides, that show neither basic nor acidic properties.
- Insoluble in Water.
- Do not react with acids or basis.
Examples:
- Carbon Monoxide (CO)
- Water (H2O)
- Nitric Oxide (NO)
- Hydrogen Per Oxide (H202)
No comments:
Post a Comment