Salts:
Salts are Ionic Compounds. A salt is formed when a metallic ion or an ammonium ion (NH4+) replaces one or more Hydrogen Ions of an acid.
A Salt contains a positive metal ion and a negative non – metal ion.
Example:
Water of Crystalization:
- Many salts combine with water molecules to form crystals . These water molecules are known as water of crystallization.
- Salts that contain Water of Crystallization are called Hydrated Salts.
- Salts that do not contain water of crystallization are called Anhydrous Salts.
Anhydrous Salts – Powder form
Hydrous Salts – Crystal / Perfectly Dry
Examples:
Copper(II) Sulphate: (Cu SO4) – CuSO4.5H20
Magnesium Sulphate (MgSO4) – MgSO4.7H20
Sodium Carbonate (Na2CO3) – Na2CO3.10H20
Zinc Sulphate (ZnSO4) – ZnSO4.7H20
Removing Water of Crystallization:
When a hydrated salt is heated, water of crystallization is given off.
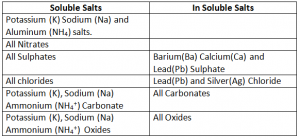
Soluble and In Soluble Salts:
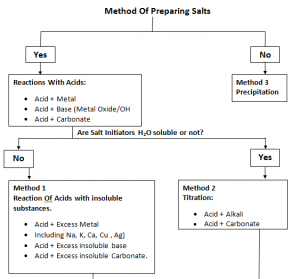
Preperation Of Salts:
- Salt that has to be made should be soluble.
- Salt starters should be soluble.
Only Acid + Alkali titration can be done.
Alkali – Soluble Base
Metal Oxides / OH Of Group # 1 and 2 as all are soluble
- Common acids used in titration:
HCl, HNO3, H2SO4
Apparatus Needed: Burette, Pipette and Conical Flask
Indicators:
Phenolphthalein:
- Acid – colourless
- Alkali – Pink
Methyl Orange:
- Acid – Red
- Alkali – Yellow
Example of Salt made through Titration:
MgSO4
Reagents / Starters:
Mg(OH)2 and H2SO4
Equation:
Explaination:
Mg(OH)2 is added by means of a pipette to a conical flask. A few drops of phenolphthalein are added. Then H2SO4 is poured into the conical flask using a burette.
The end point of the titration is reached when the phenolphthalein changes its colour from pink to colourless.
The volume of H2SO4 requires to reach the end point is noted. Then the whole procedure is repeated without addition of phenolphthalein.
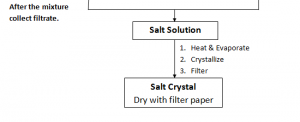
The solution is then evaporated under sunlight to form bigger crystals of MgSO4
Excessive Reagent Method:
- Salt that has to be made should be soluble.
- Salt starters should not be soluble.
Example of Salt made through Excessive Reagent Method:
Zn(NO3)
Pour 10ml of Hno3 into a beaker using a pipette. Add excessive amount of ZnO into the beaker containing HNO3.
Precipitates of excess ZnO wil form at the bottom of the beaker.
Using filteration filterate the ZnO from Zn(NO3)2 solution.
Zn(NO3)2 Solution is then heated over a burner flame in a china dish. It is then allowed to cool slowly. Crystals formed and are dried between the pieces of filter paper.
Or
Evaporate Zn(NO3)2 solution under sunlight crystals of Zn(NO3)2 will form after same time.
Precipitation:
- Salt that has to be made should not be soluble.
- Salt starters should be soluble.
Example of Salt made through Precipitation:
CaCo3
Add CaCl2 into a beaker that already contains Na2co3. Precipitates of CaCO3 will form at the bottom of the beaker. Separate the newly formed insoluble CaCO3 using filtration.
CaCO3 is then evaporated under sunlight, crystals are formed.
Key:
- All soluble salts of Group 1 and 2 except Ca sulphate and Carbonate are prepaired by titration method.
- All soluble esalts of transitional metals are prepaires by reacting the oxides with acids through excessive reagent method.
- All insoluble salts are prepaired by percepitaion method.
No comments:
Post a Comment