Matter:
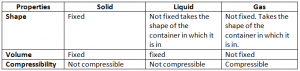
Matter is any substance that has mass and occupies space. There are three states of matter namely solid, liquid and gas.
Volume: It is the area occupied by any substance.
Compressibility: Compressibility is the effect of pressure on a substance.
Kinetic Particle Theory:
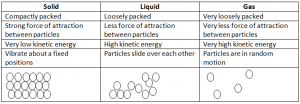
This theory states that all matter in any state comprises of tiny particles which are in continus movement.
- This theory tells that matter has three states.
- There are different properties of these three states.
- How one state of matter can be converted into another state?
The three states of matter can be explained using the kinetic particle theory.
Changes In State Of Matter:
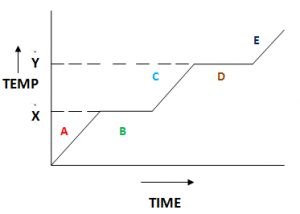
Heating Curve:
The above figure shows a heating curve of a solid. At point A the state is solid. At point B the solid is melting, at this stage it is a mixture of solid and liquid. At point C the state is liquid. At point D the liquid is evaporating, it is a mixture of liquid and gas. At point E the state is gas. Temperature X is the melting point and the temperature Y is the boiling point of the solid.
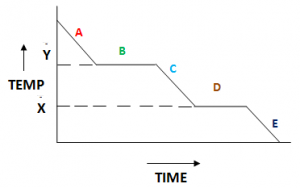
Cooling Curve:
The above figure shows a cooling curve of a gas. At point A the state is gas. At point B the gas is condensing the state over here is a mixture of gas and liquid, at point C the state is liquid. At point D the liquid is freezing at this stage there is a mixture of liquid and solid. At point E the state is solid. Temperature X is the melting point and temperature Y is the boiling point of the gas.
(Solid to liquid) (Liquid to solid)
MELTING FREEZING
(Liquid to gas) (Gas to liquid)
BOILING CONDENSATION
(Solid to gas) (Gas to solid)
SUBLIMATION DEPOSITION
Evaporation:
Evaporation is a process when the particles at top of the surface of the liquid ge more energy as compared to the particles at the bottom thus they leave the liquid and evaporate.
Evaporation only acts on the top most surface and will not affect below the top surface of the liquid.
The rate of evaporation increases if the temperature increases.
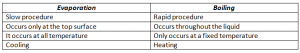
Difference Between Evaporation and Boiling:
Volatile Liquids:
Those liquids which have boiling point just above the room temperature and can evaporate very quickly. E.g. Ether (boiling point 37 0c).
Diffusion:
Diffusion is the movement of particles from higher concentration to lower concentration area.
The rate of diffusion is higher when temperature Is more as the particles at higher temperature have more kinetic energy thus they move at a greater speed.
The rate of diffusion also depends upon the molecular mass of the particle, the lighter the particle the faster it diffuses.
Evidence for Diffusion:
In Liquids: Potassium Magnate (VII) in a beaker of water.
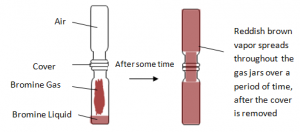
In Gases: A gas jar of air and a gas jar of Bromine connected.
Factors affecting the Rate of Diffusion:
Temperature:As temperature increases, the rate of diffusion increases. This occurs as particles at higher temperature have higher kinetic energy and thus are able to move at higher speed from region of higher concentration to less concentration.
Temperature:As temperature increases, the rate of diffusion increases. This occurs as particles at higher temperature have higher kinetic energy and thus are able to move at higher speed from region of higher concentration to less concentration.
Molecular Mass of the particle: Particles having less mass have higher rate of diffusion than those which have greater mass considering that the temperature remains constant. Therefore gases having lower density have greater rate of diffusion.
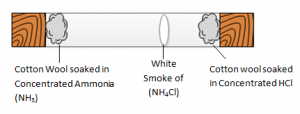
Below is an example of the reaction between aqueous Ammonia (NH3) and concentrated HCl, showing that not all gases diffuses at the same rate. The white smoke (Ammonium Chloride (NH4Cl) formed nearer to cotton wool soaked with HCl indicates that HCl molecules are heaver then NH3 molecules.
Mr NH3 = 14 + 1 * 3 = 17 Mr HCl = 1 + 35.5 = 36.5

No comments:
Post a Comment