Sulphuric Acid:
It is a colourless oily yellow liquid, with density slightly higher than water.Having a boiling point of 338°C. It is soluble in water and emits heat when dissolved in water – exothermic in nature.
Contact Process:
Sulphuric Acid is manufactured using Sulphur, Air and Water.
- Sulphur is burned in air to form Sulphur Dioxide(SO2).
SO2 is purified from impurities by passing it through dust settlers and washed with water and then dried with H2SO4. As, if impurities left the reaction would be less effective.
- Vanadium V Oxide is used as a catalyst, Platinum can also be used as a catalyst, however it is expensive.Suplhur Dioxide is cooled and further reacted with Oxygen to form Sulphur Trioxide.
- Sulphur Dioxide is dissolved in concentrated Sulphuric Acid to form Oleum.
- Upon dilution with water, Sulphuric Acid is formed.
Uses Of Sulphur Dioxide and Sulphuric Acid:
Sulphur Dioxide is used:
- In manufacturing of Sulphuric Acid.
- As a food preservative – kills bacteria.
- As a bleasching agent.
Sulphuric Acid is used:
- To make dyes.
- In production of Fertilizers.
- To make detergents.
- As an electrolyte in batteries of cars.
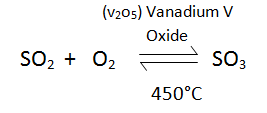
No comments:
Post a Comment