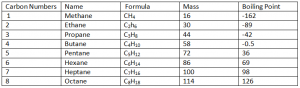
Alkanes:
Alkanes are a group of Hydro Carbons having C – C single bonds.
General Formula ( Cn H2n + 2 )
Functional Formula ( C – C )
Note: As boiling point of alkanes till Butane are below 0, therefore they are in gaseous form when at Room Temperature. On the other hand alkanes after Pentane are in liquid form at Room Temperature.
Structural Properties:
- General Formula Cn H2n + 2
- Saturated Single Covalent Bonds C – C
Physical Properties:
Insoluble in Water (H20)
As the number of Carbons increases going down the group;
- Mass, Density, Melting Point, Boiling Point increases.
- More Viscosity ( Self hindrance offered by liquid towards its flow)
- Less flammable
Chemical Properties:
- They do not react with most of the chemicals as they are saturated, having only C-C and C- H single bonds.
- They do however undergo combustion and react with chlorine in the presence of sunlight.
Combustion Reaction:
Complete Combustion:
Alkanes burn in the presence of sufficient supply of Oxygen is called complete combustion production.
HydroCarbons on complete combustion produce Carbon dioxide (CO2) and Water (H2O).
E.g.
Incomplete Combustion:
Burning in the presence of insufficient supply of Oxygen gas is called in-complete combustion.
Hydro Carbons on incomplete combustion produce Carbon Monoxide and Water.
Substitution With Chlorine:
A substitution reaction is a reaction in which one or more atoms of an organic compound are replaced with one or more other atoms.
This reaction can keep on happening (stepwise) until all the hydrogen atoms in the hydrocarbons have been replaced by a halogen (group 7 elements).
- The product produced after this reaction would be known as Halogenoalkanes.
- HCL is also the product
- Reaction speed depends upon the reactivity of the Halogen
- Reactivity of Halogen decreases down the reactivity.
- Ultra Violet radiation is providing heat energy to start up this reaction.
- Light (sunlight) is needed, to break the covalent bonds between chlorine molecule – atoms.
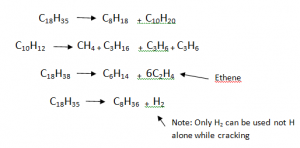
Cracking Of A Big Alkane Molecule:
- Cracking is a reaction, in which bigger Alkane/ H molecules are broken into smaller molecules. `
- The longer chain Alkanes are converted into smaller chain Alkanes, Alkenes and Hydrogen .
- The product formed after the rection, can have only Alkane or mixture of Alkane, Alkens and H2.
- Number of Carbons Remain Same, In Reactants And Products.
- Temperature requires is 600 °c
- Alumina Al2O3 and Silica SiO2 are used as catalyst.
E.g.
Uses of Cracking:
- To produce akanes and alkenes and Hydrogen.
- To breakup large hydrogen carbon molecules into smaller ones to produce fuel for motor vehicles.
- Ethene is produced, It is useful in production of Ethanol and Plastic (Polyethene).
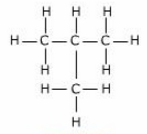
Isomerism:
- Isomers are organic compounds with the same molecular formula but different structural formula.
- Due to difference in chain length (structural properties), they have different physical properties (e.g. Boiling point, Melting point)
- They can occur in both Alkanes and Alkenes
- As the # of Carbon atoms increases, the number of isomers also increases.
- As they have same molecular formula, therefore their percentage composition by mass remains the same.
- Isomerism is used in the petroleum industry e.g. car eng
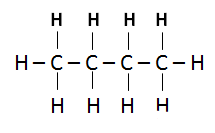
No comments:
Post a Comment