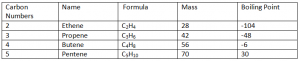
Alkenes:
Alkenes are a group of hydrocarbons, with a C=C double bond, as not all Carbon atoms are bonded to the maximum number of 4 other atoms.
General Formula: ( Cn H2n )
Functional Formula: ( C = C )
- All alkenes name ends with – ene.
- They are Unsaturated hydrocartbons
- Each member of the alkene differs from the next by a CH2
Note: As you go down the group, the mass and boiling point increases.
Branched And Unbranched Alkenes ( Isomerism in Alkenes):
- They have the same molecular formula C4H8, but have different structural formula.
- Butene is a straight-chain unsaturated hydrocarbon while methylpropen is a branched-chain, unsaturated hydrocarbon.
- Butene and methylpropene have different melting and boiling point.
Isomerism for Alkenes:
Isomers can be created in Alkenes by changing the direction of Carbon atoms or by changing the position of Carbon Carbon double bonds (C=C).
Preperation Of Alkenes ( Cracking):
- Alkenes are created by the cacking of heavier fractions of petroleum.
- High temperature and pressures are needed to break up the large molecules.
- The two catalyst used are Alumina Al2O3 and Silica SiO2 are used.
Note: More details about this topic is given in the Alkanes.
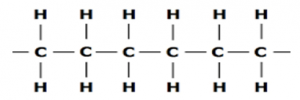
Saturated:
These are those hydro carbons, that have Carbon Carbon single bond (C-C)
In these the combining capacity of the carbon atoms is fully used as possible in bonding with Hydrogen atoms
Note: that Carbon double bond with Oxygen doesn’t make them unsaturated.
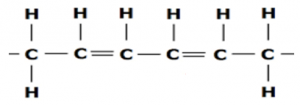
Unsaturated:
These are those hydro carbons, that have Carbon Carbon double bond (C=C).
In these the combining capacity of the Carbon atoms is not fully used e.g 2 or 3 Hydrogens are attracted to a one single Carbon atom.
Test for detecting unsaturated HydroCarbons:
Add liquid Bromine or Bromine water ( reddish brown).
If a C=C bond is present, an addition reaction takes place and the colour will be discharged.
However, in saturated compounds ( where C-C bond is present) Bromine colour will remain same.
Combustion:
All Alkenes will have more Carbon content, then their respective Alkane. Hence they would be needing more Oxygen for combustion.
Thus there is more chances of incomplete combustion.
Complete combustion of Alkenes (Excess supply of O2):
Incomplete combustion of Alkenes (limited supply of O2)
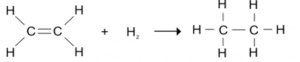
Addition Of Hydrogen / Hydrogenation:
Alkenes react with Hydrogen to form Alkanes.
- Catalyst used are Platenum (Pt), Rubidium(Rn) and Nickel( Ni)
- Temprature required is 200°C.
E.g.
As Alkene is turned into Alkane, Hydrogenation is used, to change vegetable oil, into Margarine.
Vegetable oil contains unsaturated fats with many C=C bonds, therefore it can be hardened to form margarine, the greater the Hydrogen used, the more solid(harder), the margarine becomes.
Note: Butter – higher degree of saturation (C-C bonds)
Oil – higher degree of un saturation (C=C bonds) – liquid bromine can be added to test oil, colour will change.
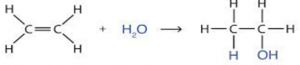
Addition Of Water (H2o)/ Hydration:
Alkenes react with steam, to produce Alcohols during hydration process.
- In this process, steam is added onto C=C Bonds in Alkene.
- Catalyst used are Phosphoric Acid (H3PO4)
- Temperature required is 300°C
- Pressure 60 atm
E.g.
Hence, hydration is used to covert Ethene into Ethanol and Propene into Propanol.
Halogenation:
Halogenation is used, to convert Alkene to Alkane
Halogen (group 7 elements) is added across the double bonds of Alkene to covert the C=C to C-C.
Note: In substitution reaction in Alkanes, Halogens were added in a step wise reaction, where as in case of Alkenes, Halogens are added up at once.
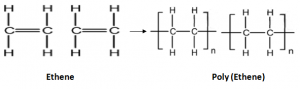
Polymerization:
Alkene molecules undergo an addition reaction, where they add on to each other to form addition Polymers. Therefore, in this reaction Alkene molecules are used as Monomers, to form addition Polymers.
Alkene molecules – monomers
Long chained compound formed – polymers
Reaction – polymerization
E.g. Ethene is polymerized to form Poly(ethane) or Polythene.
Polyethene is used to make plastic films, plastic bags and drinking bottles.
Detection Of Alkenes:
Alkenes can be detected by using cold aqueous Potassium Maganate(VII).
The purple colour of the Potassium Maganate(VII) is discharged, leaving behind a brown precipitate of Manganes (IV) Oxide MnO2 .
The Maganate(VII) ions are reduced to Manganese (IV) Ions.
Difference Between Alkenes And Alkanes:
Similarities:
- Both are compounds that only contain Carbon and Hydrogen in them.
- Both are flammable
- Form CO2 + H2O during complete combustion.
Differences:
- Molecular structure:
Alkanes C-C bonds
Alkenes C=C bonds
- Reactivity:
Alkanes are mostly unreactive.
Alkenes are more reactive than the Alkanes
- Reaction with Bromine:
Liquid Bromine when added to Alkane will not change its colour.
Liquid Bromine when added to Alkene will change its colour due to the presence of C=C bonds.
- During Combustion Alkenes produce a much smokier flame then Alkanes.
Fats And Oils:
Fats:
These are saturated fat molecules. (Solid at room temperature).
Oils:
These are un saturated fat molecules. (Liquid at room temperature).
Poly unsaturated:
These are those fats and oils whose hydrocarbon chains contain more than one C=C Bonds in their structure.
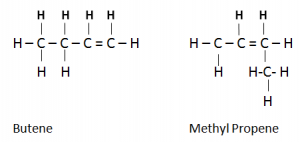

No comments:
Post a Comment