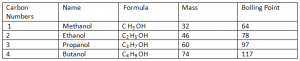
Alcohols:
Alcohols are a series of compounds with a hydroxyl group (-OH) joined to a carbon atom in a hydrocarbon chain.
General Formula: ( Cn H2n + 1 OH)
Functional Group: (OH)
Note: The above mentioned Alcohols are all in Liquid state at room temperature as their boiling > room temperature. Also moving down the group, the mass and boiling points of Alcohols increases. Alcohols and Carboxylic Acids are not HydroCarbons as they contain Oxygen atoms in their compounds.
Physical Properties:
- Alcohols are soluble in water(H2O).
- Alcohols have OH in them but they are not Alkalies.
- Alcohols are more reactive than Alkanes and Alkenes, as they have more electronic density, and presence of hydroxyl (-O-H) and carboxyl group (-C-O) as shown below.
H3C – CH3 – Ethane
H2C – CHc – Ethene
H3C – CH2 – O – H
Combustion:
Alcohol requires less Oxygen for combustion, as Oxygen is a part of it.
Complete Combustion:
Incomplete Combustion:
This reaction, is highly exothermic, thus alcohols are used as fuels.
Oxidation:
Oxidation is gain of Oxygen and loss of Hydrogen.
Alcohols upon oxidation will form Carboxylic Acids + H2O
In the presence of acidified Potassium Dichromate (H2SO4 K2Cr2O7) an oxidizing agent.
Hence:
Ethanol will become Ethanoic Acid and Propanol will become Propanoic Acid.
In this oxidation process, we can use either:
- Atmospheric Oxygen
- Acidified Potssium Dichromate , which changes its colour from Orange to Green.
- Purple acidified Potassium Magaate (VII) which is decolourised(K2Cr2O7 MNO4)
Preperation Of Alcohol:
There are two methods to obtain alcohol:
- Hydration Of Alkene (Lab Method)
- Fermentation (Industrial Method)
Hydration of Alkene (Lab Method):
In this method, steam is added to Alkene, to convert it to Alcohol.
Conditions:
- Catalyst – Phosphoric Acid (H3PO4)
- Temperature – 300 °C
Hydration of Alkene, is actually adding water (H2O) across the two double bonds Carbon of Alkenes
Fermentation (Industrial Method):
Fermentation is a process whereby an enzyme is used to break down glucose in sugar or starch into ethanol and CO2 gas.
Fermentation is catalysed by the enzymes present in yeast.
Conditions:
- Temperature – 37°C (25 – 40°C)
- Temperature too high would destroy the yeast and temperature too low would result in yeast becoming dormant.
- Catalyst/ Biological Catalyst – Yeast (enzyme)
- No Oxygen should enter the setup. As Oxygen can react with Alcohol to convert it into Carboxylic Acid.
- CO2 test (limewater turns milky ) to check the alcohol preparation.
- Alcohol can be separated after the process is completed, through fractional distillation, by using their respective boiling point.
Uses Of Alcohol:
There are different uses of Alcohols such as:
- Alcohol can be used as a constituent of alcohol beverages.
- As a solvent for detergents, perfumes and in medicines.
- As a fuel.
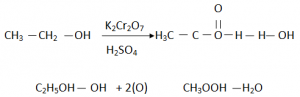
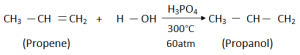

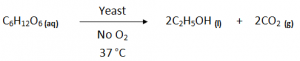
No comments:
Post a Comment