Properties Of Ammonia:
Colourless gas, with strong choking smell. It is less dense than air and reacts with hydrogen chloride gas to form a white smoke.
This reaction can also be used, to test Ammonia.
Ammonia is soluble in alkaline – reacts with acids to form salts.
Haber Process:
Haber Process is used, to manufacture Ammonia.
Nitrogen and Hydrogen is reacted together in the presence of Iron Catalyst (Fe2O3) to form Ammonia.
Equation:
The source of Nitrogen, is air – fractional ditillation of Air.
Source of Hydrogen is Methane CH4 – reacting CH4 with steam.
It is a reaversible chemical reaction doesn’t go to 100% completion.
Catalyst used – (Fe2O3).It only speeds up the reaction, doesn’t changes its yield.
Conditions :
Temperature of 450C
Pressure of 200 atm.
Ammonia as a Fertilizer:
Plants need nitrogen as on eof components for growth and ammonium fertilizers contain Nitrogen for that.
Fertilizers is any substance that is added to the soil, to make it more fertile. Plants need N2 for making Chlorophyl and proteins and hence increase the overall crop yield.
Some common Fertilizers:
Ammonium Nitrate (NH4NO3)
Potassium Sulfate (K2SO4)
Ammonium Sulfate (NH4)2SO4
Ammonium Phosphate (NH4)3PO4
Reactions to make Fertilizers:
Comparing Nitrogen Content:
Different Fertilizers are compared, in order to see which fertilizer contains more Nitrogen.
Lets Compare Ammonium Sulphate (NH4)2SO4 with Urea (NH2)2CO
Ammonium Sulphate (NH4)2SO4:
21.2% of Nitrogen
Urea (NH2)2CO:
46.7% of Nitrogen
Hence Urea (NH2)2CO is a better fertilizer since it contains more Nitrogen.
Eutriphication:
Nitrates are highly soluble in water. Hence when used as a fertilizer for crops, excessive amount of nitrate travels with water to the river and canals. Nitrate is helpful for the growth of Algae. Algae covers the whole area thus blocking sunlight and oxygen to aquatic plant and life. Moreover, if someone drinks that water – its contents mixes with hemoglobin and may cause severe illness.
Uses Of Ammonia:
- Ammonia is used in making Fertilizers – that help in increasing crop yield.
- Used in manufacturing of Nitric Acid.
- Production of explosives and nylon.
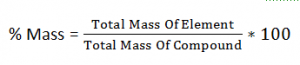
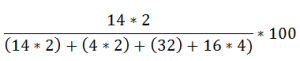
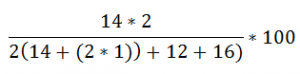
No comments:
Post a Comment