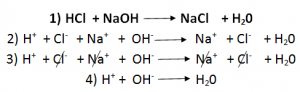
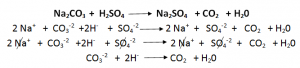
Writing Ionic Equations:
An ionic equation is a simplified chemical equation that shows the reactions of ionic compounds in water.
Ionic compounds are those compounds, which are soluble in water.
Inorder to write an ionic equation:
- Write the balanced chemical equation of the reaction. Include the state symbols.
- Identify ionic compounds that are soluble in water. These compounds become ions in H20. Rewrite the chemical equation in terms of ions.
- Cancel out the spectator ions (Common).
- Write the ionic equation.
Relative Atomic Mass (Ar):
The mass of an atom compared with the Carbon – 12 atom is called its relative atomic mass.
The mass of one mole of atoms is its “relative atomic mass” in grams.
Relative Molecular Mass (Mr):
The mass of a substance made of molecules is known as Relative Molecular Mass.
Hydrogen has (1*2) = 2 and H20 has relative molecular mass of (1*2) + 16 = 18
Relative Formula Mass (Mr):
The mass of a substance made of ions is known as relative formula mass. Ammonia (NH3) has Mr of (1*14) + (3*1) = 58.5.
The Mole:
A mole of a substance is the amount that contains the same number of units as the number of Carbon atoms in 12 grams of carbon-12.
Avogradoe’s Number:
Number of Particles in one mole = 6.02 * 1023
Percentage Compostition of Compounds:
Percentage by mass of an element in a compound
Example:
Percentage of Hydrogen in Hydrogen Per Oxide:
Water in Coppoer(II) Sulphate:
Emperical Formula:
Emperical Formula of a compound shows:
- Types of elements present in it.
- Simplest Ratio of the different types of atoms in it.
Inorder to Find the Emperical Formula of a compound:
- Write down the each percentage/ mass separately.
- Divide each by their Mr.
- Now divide all of them with one which has the lowest ratio.
- If any one of the answer is in the decimal form, multiply both with any number (lowest) to get make it a whole number
Examples:
A compound has 40% Carbon 6.6% Hydrogen & 53.3% Oxygen. Calculate the Emperical Formula of the compound.
C H O
40 6.6 53.3
40/12 6.6/1 53.3/16
3.33 6.6 3.33
3.33/3.33 6.6/3.33 3.33/3.33
1 2 1
Emperical Formula: CH2O
E.g 2 O.72g of Mg combines with 0.28g of Nitrogen. Find its Emperical Formula.
Mg N
0.72 0.28
0.72/24 0.28/14
0.03 0.02
0.03/0.02 0.02/0.02
1.5 1
1.5 * 2 1 * 2
3 2
Emperical Formula :Mg3N2
Molecular Formulae:
The molecular formula shows the actual number of atoms that combine to form a molecule.
To Find the molecular Formula:
- Calculate
 for the compound. This gives the number n.
for the compound. This gives the number n. - Multiply the numbers in the empirical formula n.
E.g. Emperical Formula = HO
Relative Molecular Mass = 34
(H = 1 , O = 16) . Find Molecular Formula?
34/17 = 2
HO * 2 = H202
Calculating The Volume of Gas:
1 Mole of every substance occupies 24dm3.
The Concentration of a solution, is the amount of solute in grams or noles, that is dissolved in 1 dm3 of solution.
Percentage yield:
Yeild is the amount of product, obtained from a reaction.
Actual Yeild:
It is the amount collected at the end of a chemical reaction. The actual yield is always less than the theoretical yield. It is also known as the Practical Yeild.
Theoratical Yeid:
It is the calculated yield of the amount of prosuct by using stoichiometry. In this Yeild 100% reactants are converted to products, with no losses.
Percentage Purity:
Percentage Purity indicates the amount od pure substance present in a sample of chemical substance.

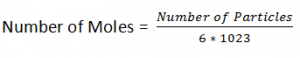
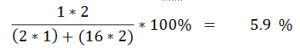
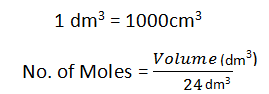

No comments:
Post a Comment