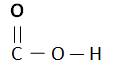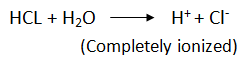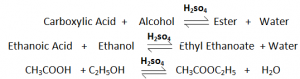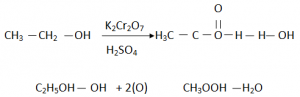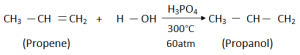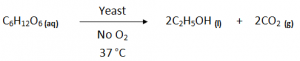For the past papers click here:
http://papers.xtremepapers.com/index.php?dir=CIE/Cambridge%20International%20O%20Level/Chemistry%20%285070%29/
O-LEVEL CHEMISTRY
Friday, 19 May 2017
Macro Molecules
Macro Molecules:
Macro Molecules, are large molecules, made by joining together of many small molecules.
This process of joining together smaller units into a large macro molecule polymer is called polymerization.
Monomer:
Small molecules, that join together to form one large polymer molecules.
There are two kinds of polymer:
Synthetic Polymer
Natural Polymers
Synthetic Polymers:
Synthetic Polymers are manmade polymers that are formed by
- Addition Polymerization – Addition Polymers
- Condensation Polymers – Condensation Polymers
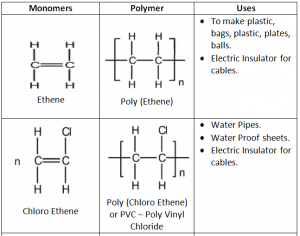
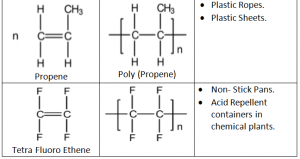
Addition Polymers:
Addition Polymers are made from unsaturated monomers through an addition reaction.
Repeating Unit: Simplest part of the polymer which is repeated many times to form the polymer.
In addition Polymerization any alkene is converted into Poly alkene.
Alkane is converted to Alkane.
Making of Poly Ethene conditions:
Temperature – 200 °C
Atmospheric Pressure: 1000
Catalyst – Zeigler’s Catalyst – TiCl4 + (C2H5)3 Al – Mixture of Titanium Tetre Chloride and Tetra Aluminium
Further Advantages of Addition Polymerization:
Perspex – Used in making car windows
Taflon – Used in production of utensils, rain coats and rubber tubings
Polystylene – Disposable glasses and plastics.
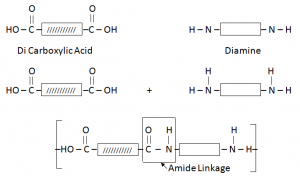
Condensation Polymers:
When two different monomers join, each with two functional group. The monomers join their functional groups, by getting rid of or eliminating small molecules.
Condensation Polymers are made from monomers containing alcohol, acid or amino functional groups.
Through elimination of small molecules like H2O. E.g. Nylon and Teryline.
Nylon/ Polyamide:
It is formed by the combination of Di Carboxylic Acid and Di Amine.
- Why Nylon also called as Poly Amide – due to presence of Amide Linkage.
Uses of Nylon:
- Nylon is used in making synthetic fibres
- In production of ropes, fishing lines and clothes
- For making rain coats and parachutes.
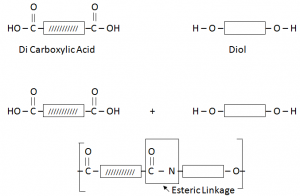
Terylene / Polyester:
Terylene is formed by the combination of DiCarboxylic Acid and Diol (Hydroxyl Group).
Uses of Terylene:
- Fabrics – That are strong, resists strechingand sinking. Doesn’t crumple.
- Sleeping bags are made using teryline. Hence they do not shrink (Synthetic Fibre).
Disadvantages of Plastic:
- Plastics are non-biodegradeble, can’t be decomposed by bacteria. Hence they result in polluting the earth.
- Produce toxic gases(HCl) when burnt, this contriburtes to acid rain.
- Plastics are Carbon based polymers – burn easily.
- Plastics that require Chloro-Flouro Carbons (CFC) during production may contribute to global warming when the CFC is allowed to escape. Thus may lead to flodding in low lying areas.
Natural Polymers:
Natural Polymers are formed by condensation polymerization.
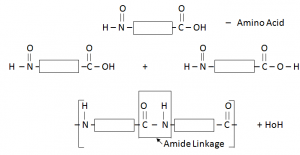
Protein:
When two amino acids combine with each other, protein is formed and H2O is a by-product.
Protein have similar linkage to that of Nylon (amide linkage) –Thus can be called Polyamide.
They are joined together by condensation polymerization.
Each amino acid has an acidic functional group and an amino functional group.
Proteins are possessing the same amide linkages as Nylon but with different monomer units.
Proteins can also by hydrated back into amino acids, by heating them with Sulphuric Acid (H3PO4).
Hydrolysis:
Is a reaction in which molecules are broken down by reaction with water, in the presence of Alkali or Acid.
The products of the hydrolysis of protein (amino acids) and Carbohydrates can be separated and identifies through chromatography.
The chromatogram needs to be sprayed with a locating agent (Ninhydrin) so that they can become visible and be compared to other pure samples of the monomer.
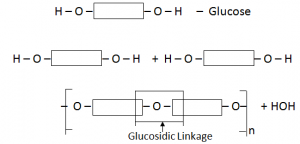
Carbohydrates:
Carbohydrates are polymers made up of small sugar molecules joined together e.g. starch.
Carbohydrates contain Carbon, Hydrogen and Oxygen.
General Formula: CnC(H2O)n
Simplest Carbohydrate C6H12O6 (Glucose)
Glucose polymerise each other to form starch
Equation:
C6H12O6  (C5H10O5)n + nH2O
(C5H10O5)n + nH2O
Hydrolysic of Carbohydrates to convert them to simple sugar:
Starch can be broken down to glucose (Simple Sugar molecules by heating with Sulphuric Acid (Hydrolysis) – H2O molecule is added.
Chromatography can be used to identify small molecules of sugar
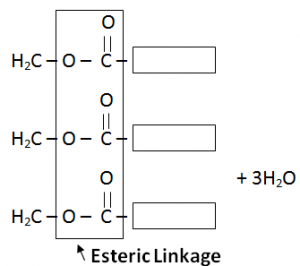
Fats:
Fats are formed through condensation reaction of fatty acids (Carboxylic Acids) and Glycerol (3 Carboon atom chains having OH attached with every Carbon atom).
H2O is also a by – product.
Fat is formed as a single large molecule so no need to write continuation brackets.
Glycserol contains three – OH functional groups per molecule and is hence known as a triol.
Each molecule of glycerol will combine with three molecules of fatty acids and form one molecule of fat.
Fats have same esteric linkages as Terylene (also known as polyster).
Fats can also be broken down to sodium salts of fatty acids and glycerol by boiling it with Acid or Alkalis (Hydrolysis).
Carboxylic Acids
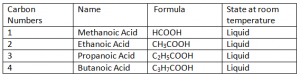
Carboxylic Acids:
Carboxylic Acids are homogenous Series/ organic compounds containing the (CO2H) group.
General Formula: CnH2n + 1 COOH
Carboxylic Acids are not HydroCarbons, as they contain Oxygen in their Compounds. Hydro Carbonds are those organic compounds containing only Hydrogen and Carbon.
Properties Of Carboxyic Acids:
- All of them are 100% soluble in water.
- Chemical reaction of Carboxylic Acids are dependent upon C = O (Carboxyl) and H = O (Hydroxyl).
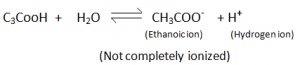
- They are weak acids (partially ionizes in water).
i.e. weaker than inorganic acids – inorganic compounds completely ionize hence more reactive than carboxylic acids.
In Organic Acid:
Organic Acid:
- Carboxylic Acids are partially ionized, to give Hydrogen ions in H2
- They dissacociate only partially in water to form hydrogen ions.
Reactions With Metals:
Reactions With Carbonates + Bi Carbonates:
Reactions With Basis:
Production Of Ehtanoic Acid (Carboxylic Acid):
Ethanoic Acid is dropped by the oxidation of Ethanol.
- Using Atmospheric Oxygen:
- By the oxidation of Ethanol in fermented solution with atmospheric oxygen in the presence of certain bacteria.
- When ethanol is left standing in air, bacteria bring about its oxidation to Ethanoic acid – acid fermentation.
- Acid fermentation is used to make vinegar (a dilute solution of Ethanoic acid.) The vinegar is produced from foods such as apples, rice and honey which are first fermented to give ethanol.
C2H5OH(l) + O2(g) CH3COOH(l) + H2O(l)
- Using Oxidizing Agents:
- By heating Ethanol with an oxidizing agent such ad acidified Potassium Dichromate(VI) (K2Cr2o7).
- Ethanol is oxidized much faster by warming the oxidizing agent K2Cr2o7 in the presence of an acid.
- K2Cr2o7 produces atomic Oxygen which more reactive than normal Oxygen (O).
- The Orange K2Cr2o7 solution changes its colour to Green.
- H2O is also produced as a byproduct while producing ethanol.
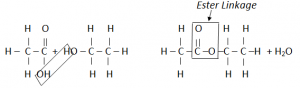
Esterification:
Esters are organic compounds formed by the combination of Carboxylic Acis with an Alcohol.
Catalyst – Concentrated Sulphuric Acid (H2SO4)
This Reaction is reversible.
H20 is produced as a by – product.
Ester is a sweet smelling organic compound.
Preperation of Ester is known as esterification.
We can add Sodium Hydroxide (NaOH) and heat mixture to obtain Carboxylic acid and alcohol from ester – This is Hydrolysis.
OH – from acid H from alcohol – Ester formation
The two functional groups react with each other. The C of the Carboxyl group bonds to the O of the OH group. The COO group where they join is called ester linkage.
The two molecules have joined by getting rid of a small molecule, water. This is called condensation reaction.
In Condensation reaction, two molecules join together to form a large molecule, with the loss of a small molecule.
Uses of Esters:
- As a flavouring agent in foods.
- Used in cosmetics.
- Used as a solvent, to dissolve organic compounds that are insoluble in wate. They are volatile, hence they evaporate easily. (painting, ink, glues and nail polish).
- Esters are used in creating vegetable oils.
Alcohols
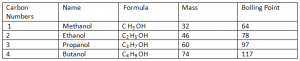
Alcohols:
Alcohols are a series of compounds with a hydroxyl group (-OH) joined to a carbon atom in a hydrocarbon chain.
General Formula: ( Cn H2n + 1 OH)
Functional Group: (OH)
Note: The above mentioned Alcohols are all in Liquid state at room temperature as their boiling > room temperature. Also moving down the group, the mass and boiling points of Alcohols increases. Alcohols and Carboxylic Acids are not HydroCarbons as they contain Oxygen atoms in their compounds.
Physical Properties:
- Alcohols are soluble in water(H2O).
- Alcohols have OH in them but they are not Alkalies.
- Alcohols are more reactive than Alkanes and Alkenes, as they have more electronic density, and presence of hydroxyl (-O-H) and carboxyl group (-C-O) as shown below.
H3C – CH3 – Ethane
H2C – CHc – Ethene
H3C – CH2 – O – H
Combustion:
Alcohol requires less Oxygen for combustion, as Oxygen is a part of it.
Complete Combustion:
Incomplete Combustion:
This reaction, is highly exothermic, thus alcohols are used as fuels.
Oxidation:
Oxidation is gain of Oxygen and loss of Hydrogen.
Alcohols upon oxidation will form Carboxylic Acids + H2O
In the presence of acidified Potassium Dichromate (H2SO4 K2Cr2O7) an oxidizing agent.
Hence:
Ethanol will become Ethanoic Acid and Propanol will become Propanoic Acid.
In this oxidation process, we can use either:
- Atmospheric Oxygen
- Acidified Potssium Dichromate , which changes its colour from Orange to Green.
- Purple acidified Potassium Magaate (VII) which is decolourised(K2Cr2O7 MNO4)
Preperation Of Alcohol:
There are two methods to obtain alcohol:
- Hydration Of Alkene (Lab Method)
- Fermentation (Industrial Method)
Hydration of Alkene (Lab Method):
In this method, steam is added to Alkene, to convert it to Alcohol.
Conditions:
- Catalyst – Phosphoric Acid (H3PO4)
- Temperature – 300 °C
Hydration of Alkene, is actually adding water (H2O) across the two double bonds Carbon of Alkenes
Fermentation (Industrial Method):
Fermentation is a process whereby an enzyme is used to break down glucose in sugar or starch into ethanol and CO2 gas.
Fermentation is catalysed by the enzymes present in yeast.
Conditions:
- Temperature – 37°C (25 – 40°C)
- Temperature too high would destroy the yeast and temperature too low would result in yeast becoming dormant.
- Catalyst/ Biological Catalyst – Yeast (enzyme)
- No Oxygen should enter the setup. As Oxygen can react with Alcohol to convert it into Carboxylic Acid.
- CO2 test (limewater turns milky ) to check the alcohol preparation.
- Alcohol can be separated after the process is completed, through fractional distillation, by using their respective boiling point.
Uses Of Alcohol:
There are different uses of Alcohols such as:
- Alcohol can be used as a constituent of alcohol beverages.
- As a solvent for detergents, perfumes and in medicines.
- As a fuel.
Subscribe to:
Comments (Atom)